Potential of hydrogel treatment in forest regeneration: impact on growth and vitality of Central European tree species
- Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Prague, Czechia
Introduction: Drought negatively affects the growth and yield of plants. Several measures have been employed to improve the drought tolerance of plants, including the application of superabsorbent hydrogel (SAH) to soil. However, studies on the effect of SAH on trees in Central Europe, which has a temperate climate, are scarce.
Methods: Here, the effects of SAH treatment on the cultivation of four key tree species in Central European forest ecosystems—European beech, sessile oak, Scots pine, and Norway spruce—were evaluated. Field and greenhouse experiments were conducted; the greenhouse conditions served as the reference for the absence of water stress.
Results and Discussion: SAH treatment reduced seedling mortality by 1%–9% in the field experiment. The morphological parameters of the control and SAH-treated seedlings did not significantly differ. Among the tree species, oak seedlings exhibited a positive response to SAH treatment. Spruce, pine, and beech did not respond to SAH treatment; the proline content of SAH-treated conifer leaves was three times higher than that of oak leaves but still very low, revealing no drought stress. The results highlight the significance of employing an individual approach in the application of SAH in forestry, rather than relying on generic measures.
1 Introduction
Recently, forest ecosystems worldwide have been affected by drastic changes in the climate, including changes in temperature, precipitation, and humidity (Choi et al., 2011; Marchi et al., 2018). The climate in Central Europe tends to decrease the soil-water content of the land between April and September (Brázdil et al., 2015); however, the total precipitation tends to increase in all seasons, except for the summer. The discrepancy between soil dryness and increasing precipitation is attributed to a higher evaporative demand as the temperature increased by 0.7°C from 2011 to 2019 (Maitah et al., 2021). Climate change is expected to increase the severity and frequency of drought in Central Europe, especially in the southern regions (Hungary and south Slovakia) (Hlásny et al., 2014).
One of the key challenges in forest management in the future is maintaining the genetic diversity of trees and altering tree species composition during forest regeneration to improve the resistance of forest ecosystems to climate change and extreme weather conditions, such as drought during the initial growth of trees (Hlásny et al., 2014). The hybridization of drought-tolerant tree species and assisted migration of trees have been proven beneficial in improving the drought tolerance of trees; such programs are being applied in Canada and Mexico (Sáenz-Romero et al., 2020). Forest managers also employ traditional measures, such as drought hardening of seedlings in the nursery and using containerized seedlings for transplanting. Adding soil conditioners (organic substrate, mycorrhizae, or synthetic polyacrylamide), especially superabsorbent hydrogels (SAH), one of the most promising additives, during forest regeneration can help to mitigate transplanting shock and increase the survival of newly planted forests (Beniwal et al., 2011).
SAH has a hydrophilic three-dimensional network (Saha et al., 2020), which affects soil permeability, texture, rate of evaporation from the soil, and rate of water infiltration into the soil (Ekebafe et al., 2011); thus, applying SAH can improve soil structure, increase water retention, and increase water availability to plants. Commercially available SAHs have a water absorption capacity ranging from 100 to 500 g g−1 SAH (Akhter et al., 2004; Koupai et al., 2008; Saha et al., 2020). Plants can survive through a prolonged dry period as the soil surrounding the rhizosphere begins to dry out, because they continue absorbing water and nutrients from the hydrogel (Hüttermann et al., 1999). However, an excessive amount of SAH may yield adverse effects, including the drying out of plant roots (Watanabe et al., 2019), as SAH pulls out water from the root tissue in the case of an overdose or dried-out soil. In terms of alleviating drought stress in plants, mycorrhizae have elicited positive effects on plant growth compared with SAH (Rydlová and Püschel, 2020). One of the negative effects of SAH application could be the swelling of hydrogel granules, which pushes seedlings out of the soil after precipitation, as evidenced by an approximately 18% decrease in the survival of rate of Pinus sylvestris L. after the application of dry SAH to the pit (Sarvaš et al., 2007). SAH also interacts with the chemical properties of soil, as high ion concentrations in fertilizers and soil reduce the monomer polymerization and water absorption of SAH (Zhong et al., 2013).
The physiological effect of SAH on forest tree species is rarely evaluated. Based on the results of the experiments conducted so far, it has been concluded that the addition of SAH to slowly dissolving fertilizers improves plant growth (Jungsinyatam et al., 2022). However, this effect has mainly been observed in semi-arid, drought-exposed, or controlled laboratory conditions, with both soil and climatic conditions vastly different from those of the forests in Central Europe. Moreover, previous studies have focused on tree species, such as mango or mandarin trees, Siberian elm, silver maple, and others, with very limited distribution and low growth potential in Central Europe and their forest management (Kargar et al., 2017; Hamdy et al., 2020; Alshallash et al., 2022). Here, we conducted field and greenhouse experiments involving the application of SAH, with an emphasis on the key tree species of Central European forests—sessile oak Quercus petraea (Matt.) Liebl., European beech Fagus sylvatica L., Norway spruce Picea abies (L.) H. Karst., and Scots pine Pinus sylvestris—to evaluate the impact of SAH on the morphological and physiological parameters of seedlings under the current temperate climatic conditions in Central Europe (i.e., without prolonged or extreme drought). We determined the effects of SAH treatment on seedling vitality and growth in the absence of drought stress and the responses of each tree species based on their physiological and morphological traits.
2 Materials and methods
2.1 Greenhouse experiment
A completely randomized design was applied in the greenhouse experiment involving the four tree species: F. sylvatica (beech), Q. petraea (oak), Pinus sylvestris (pine), and Picea abies (spruce), and two treatments (SAH and control) (Supplementary Table S1). The beech, oak, and spruce seedlings came from the Bohemian Upland area, and pine seedlings were obtained from Polabí at altitudes between 400 and 700 m a.s.l. Two-years-old bare root seedlings from the forest nursery were planted in pots containing 9 dm3 of peat and fertilizer in March 2020. SAH (STOCKOSORB® 660, Evonik Nutrition & Care GmbH, Germany) was added in the form of 1 g of fine powder mixed in 200 mL of water to the planting hole beneath the seedling roots during transplanting. The SAH, according to the manufacturer, consisted of acrylic acid, homopolymer, and potassium salt. As the peat did not contain any nutrients, commercially available SILVAMIX® fertilizer (SILVAMIX® R 30 TE; ECOLAB ZNOJMO, Znojmo, Czech Republic) that contained the essential macronutrients [total nitrogen (N), 11%; urea formaldehyde nitrogen (N), 7%; urea formaldehyde nitrogen (N) soluble in cold water, 2.8%; nitrogen from urea formaldehyde (N) soluble only in hot water, 2.7%; urea nitrogen (N), 4.0%; total phosphorus oxide (P2O5), 17%; phosphorus oxide (P2O5) soluble in neutral ammonium citrate and water, 14% and 12%, respectively; water-soluble potassium oxide (K2O), 8%; and total magnesium oxide (MgO), 7%] was applied. The fertilizer was diluted 1:10 with water and applied every 14 days throughout the experimental period.
The basic soil water constants—field capacity (FC), refill point (RP), and permanent wilting point (PWP)—were determined for the peat using the pressure-plate apparatus at corresponding pressure potentials of −20, −300, and −1,500 kPa, respectively. A volumetric soil moisture sensor (VIRRIB®, FIEDLER, Czech Republic) was used to monitor the volumetric moisture of the soil in the pots (two sensors for each treatment and each species) and the seedlings were automatically drip-irrigated to maintain the water content of the peat at approximately 40%, which roughly corresponded with the FC of the peat. A total of 512 trees were planted, and each combination of hydrogel treatment or control and tree species was represented by 64 trees. The research was conducted from March to November 2020.
During the experiment, the plants were maintained under optimal humidity, light, and nutritional conditions to avoid seedling mortality and to set baselines for all control and SAH-treated seedlings. The average temperature was 19.1 ± 6.1°C and the relative air humidity was 74.6% ± 20.3 during the first growing season. Although photosynthetically active radiation was reduced by a plastic film that covered the greenhouse and prevented direct radiation, the values exceeded the saturation point of photosynthesis (approximately 450 μmol m2 s−1).
First, the physiological measurements—photosynthetic rate (Pn) and transpiration rate (Tr)—were taken directly in the greenhouse using a gasometric system. The proline concentration was later estimated in the laboratory, as described in the analytical methods chapter. All data were taken simultaneously together with other information concerning the leaf area, internal temperature, external photosynthetic active radiation, etc. The measurements were performed on leaves for broadleaved trees and needles for conifers, with the necessary needle area measured. All measurements were performed on the annual shoots of seedlings.
Second, the samples of needles or leaves were removed with scissors and put in liquid nitrogen in a Dewar flask immediately; these samples were used for proline analysis. In the laboratory, the needles or leaves were removed from the falcon tube and 100-mg samples were weighed. After weighing, the material was added to an Eppendorf tube with three beds and then into the mill. Each treatment was represented by six replicates seedlings.
2.2 Field experiment
In 2019, a study site was established in a forested area near Kostelec nad Černými lesy (49°56.37′ N, 14°20.96′ E), Central Bohemia, Czech Republic. The site has a flat relief and the bedrock is predominantly composed of sandstone and conglomerate rock, with loess clay soil. The long-term average precipitation in the area (1980–2016) is 697 mm and the average temperature is 7.7°C (Podrázský et al., 2009). During the experiment, no severe heat events were recorded and, although precipitation was not normally distributed throughout the vegetation season of 2020, no extreme drought was recorded (Supplementary Figure S1).
A completely randomized design was followed. Two-years-old bare root seedlings of F. sylvatica, Q. petraea, P. sylvestris, and P. abies from the same provenance used in the greenhouse experiment were planted in a mixed manner in March 2019. The seedlings were obtained from a commercial forest nursery and all seedlings were transplanted on the same day. More detailed information about the seedlings planted is presented in Supplementary Table S1. Each planting hole received one of the two treatments: without SAH application (control) and with SAH. SAH (STOCKOSORB® 660, Evonik Nutrition & Care GmbH, Germany) was added in the form of 1 g of fine powder mixed in 200 mL of water to the planting hole beneath the seedling roots. In total, 400 seedlings of each species were planted: 200 were treated with SAH and 200 were retained as a control sample. The seedlings were not irrigated or fertilized after transplanting.
The physiological parameters and proline content of the seedlings were analyzed following the same design applied in the greenhouse experiment.
2.3 Analytical methods
2.3.1 Morphological parameters and gasometric measurement
In May and September 2020, the following measurements of the seedlings were taken: height, diameter of the root collar measured by a digital caliper, vitality, and related measurements of physiological characteristics. Vitality measurements were performed for all seedlings in individual categories:
1. Excellent to slightly reduced vitality,
2. Clearly reduced vitality (stagnant growth and drying of the youngest shoots),
3. Residual vitality (most of the crown is dead), and,
4. Dead seedling.
The assimilation rate (Pn), transpiration rate (Tr), and water use efficiency (WUE) were calculated based on the light curve monitored using a gas exchange system (LI-6400XT; LI-COR, Inc., Nebraska, United States); all variables were calculated with maximum light at 1,500 μmol m−2 s−1. For each treatment and tree species, six seedlings were physiologically evaluated; these measurements were taken from different seedlings than those used for the proline analyses. An artificial diode (6400-02B-LED) was applied to generate uniform and stable light inside the chamber. The temperature was adjusted according to the external conditions. A standard light curve was plotted for the fully developed leaves. The reference CO2 concentration was set at 415 μmol m−2 s−1 and the light gradually decreased from 1,500 to 0 μmol m−2 s−1.
2.3.2 Proline content
A ninhydrin-based method was used for proline estimation using a cuvette spectrophotometer (Lee et al., 2018). The leaves or needles of six selected trees from both experiments (greenhouse and field), every treatment, and every species were stored in the freezer at −80°C. Proline was extracted from 100 mg of the needles or leaves in 1.5 mL of sulfosalicylic acid. The samples were homogenized into powders using an oscillating mill. The extract was centrifuged (Model 1-16K; Sigma, Germany) for 5 min at 13,500 rpm. After centrifugation, 400 μL of the supernatant was added to Eppendorf tubes containing 400 μL of acetic acid and 400 μL of ninhydrin. After repeated centrifugation, a heat block was used to heat the mixture to 95°C for 1 h. After the mixture had cooled, 800 μL of toluene was added and the preparation was thoroughly mixed. A UV–vis spectrophotometer (DR6000; Hach Company, Colorado, United States) was used to measure the absorbance at 520 nm.
2.4 Statistics
A Kruskal–Wallis test was run in the TIBCO Statistica® 14.0.1 software to compare the measured seedling parameters between the SAH and control groups. The significance level of the factors was set at α < 0.05. The data collected from the field and greenhouse experiments were analyzed using the same statistical methods.
3 Results
3.1 Basic physiological parameters
3.1.1 Greenhouse experiment
The application of SAH significantly impacted several physiological traits in the studied tree species. SAH positively influenced the main physiological traits of oak seedlings (Figures 1A–D). The net photosynthesis (Pn) was significantly higher with the SAH treatment (Figure 1B). The favorable growth conditions, together with SAH treatment, also resulted in a higher rate of transpiration (Tr), although the difference in the transpiration rate and water use efficiency (WUE) between treatments was not significant (Figures 1C,D). The quantum yield of photosynthesis, which represented the tangent to the light curve, was significantly higher in the SAH group (0.08 ± 0.01 mol O2 mol−1) than that in the control (0.06 ± 0.01 mol O2 mol−1), indicating that the conversion of light into fixed carbon had improved (Figure 1A). On the contrary, the Tr of the control beech (Figure 1G) and pine (Figure 1O) seedlings was higher than that of the SAH group; additionally, the SAH-treated beech seedlings had a significantly lower WUE (Figure 1H). The Pn and quantum yield of photosynthesis in spruce seedlings was also higher in the control group than that in the SAH groups (Figures 1I,J).
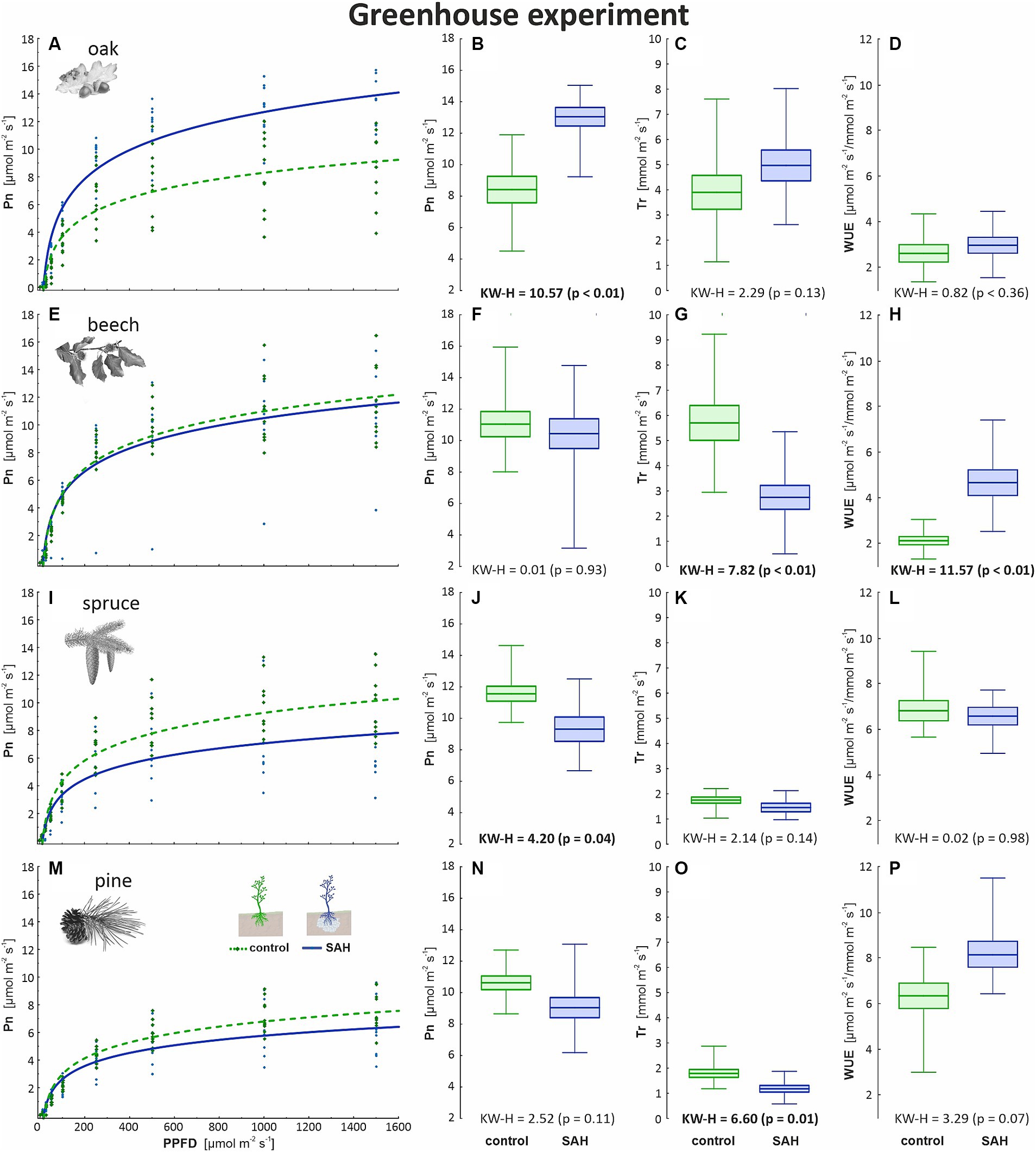
Figure 1. Measured physiological parameters in the greenhouse experiment involving superabsorbent hydrogel-treated (SAH) and control seedlings. Light curve describing the course of CO2 assimilation (Pn) dependent on the incoming light (PPFD—photosynthetic photon flux density) in (A) oak, (E) beech, (I) spruce, and (M) pine; net photosynthesis (Pn) for maximum light at 1,500 μmol m−2 s−1 in (B) oak, (F) beech, (J) spruce, and (N) pine; rate of transpiration (Tr) in (C) oak, (G) beech, (K) spruce and (O) pine; and water use efficiency (WUE) in (D) oak, (H) beech, (L) spruce, and (P) pine. Horizontal lines indicate the mean, box indicates standard error, and whiskers indicate the minimum and maximum values; KW–H represents the Kruskal–Wallis test results—significant differences are in bold.
3.1.2 Field experiment
The results from the field and greenhouse experiments were congruent, as positive trends in Pn, Tr, and WUE in oak were recorded (Figures 2A–C), although the difference in Pn was not significant (Figure 2B). Furthermore, the quantum yield of photosynthesis was higher in the SAH-treated seedlings of both spruce and pine (Figures 2I,M). Overall, increased variability of the measured parameters in the field was observed but no clear trend was found for the SAH treatment in spruce and pine (Figures 2E–P). Almost identical data were recorded for all measured parameters in the control and SAH-treated beech samples (Figures 2E,F,G,H).
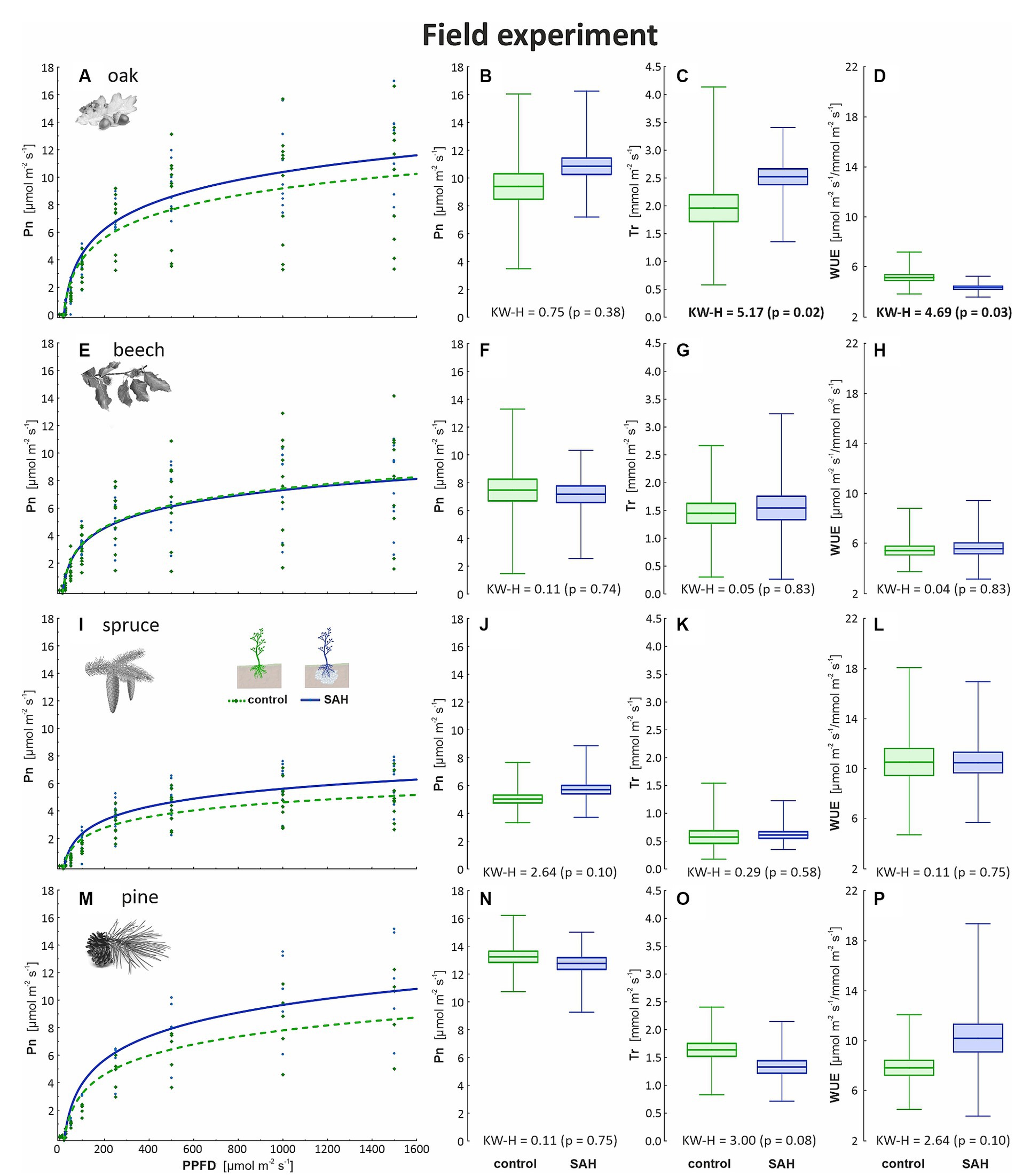
Figure 2. Measured physiological parameters in the field experiment involving superabsorbent hydrogel-treated (SAH) and control seedlings. Light curve describing the course of CO2 assimilation (Pn) dependent on the incoming light (PPFD—photosynthetic photon flux density) in (A) oak, (E) beech, (I) spruce, and (M) pine; net photosynthesis (Pn) for maximum light at 1,500 μmol m−2 s−1 in (B) oak, (F) beech, (J) spruce, and (N) pine; rate of transpiration (Tr) in (C) oak, (G) beech, (K) spruce, and (O) pine; and water use efficiency (WUE) in (D) oak, (H) beech, (L) spruce and (P) pine. Horizontal lines indicate the mean, box indicates the standard error and whiskers indicate the minimum and maximum values; KW–H represents the Kruskal–Wallis test results—significant differences are in bold.
3.2 Proline content
3.2.1 Greenhouse experiment
As drought stress was not induced in the greenhouse, the proline concentration of all investigated species was below 1 μmol g−1 of the fresh weight (FW). Regardless of the normal conditions, statistical differences were observed among the oak seedlings in the greenhouse experiment (Figure 3A). Additionally, the proline content of SAH-treated spruce was higher, although the difference was not significant (Figure 3C). Overall, beech (Figure 3B) and pine (Figure 3D) had the lowest and highest proline concentrations, respectively. The proline content of the control and SAH-treated beech was less than 0.01 μmol g−1 of FW, whereas that of pine reached 0.14 ± 0.04 μmol g−1 of FW for both treatments.
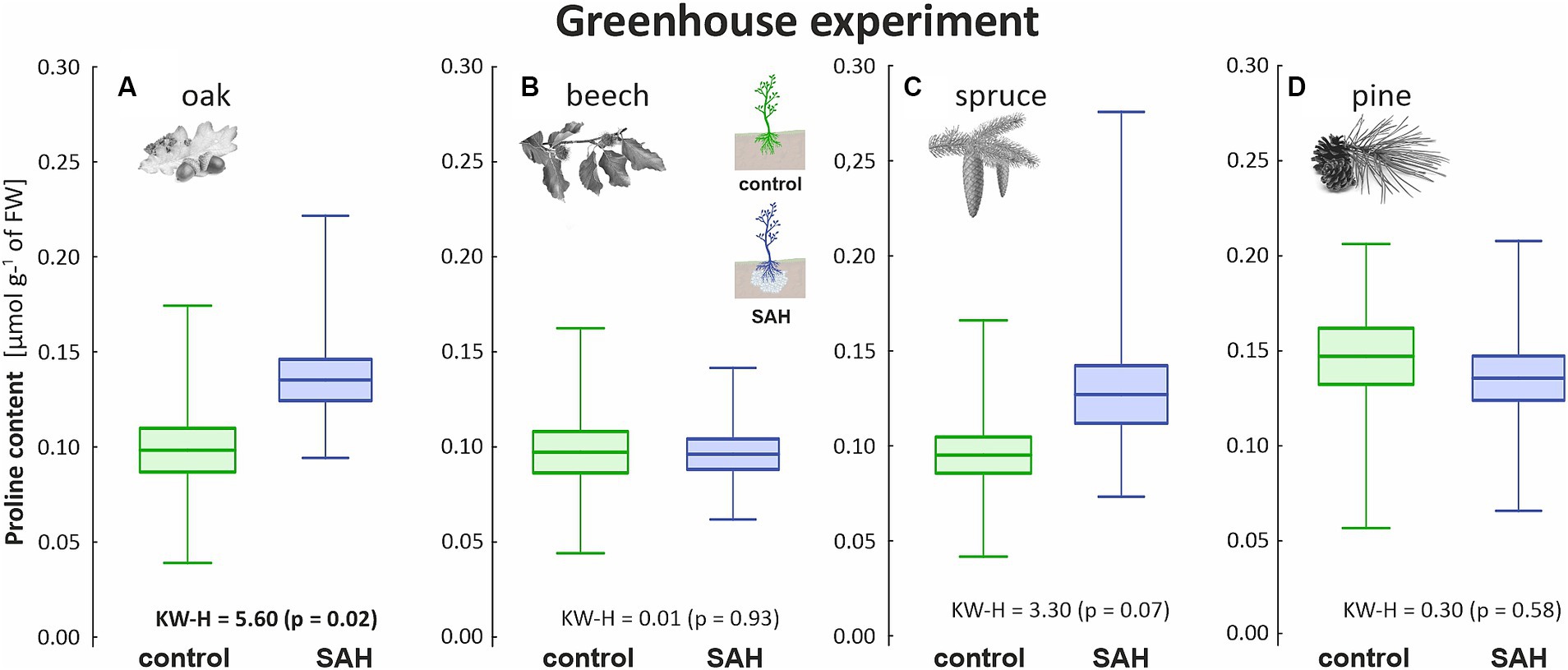
Figure 3. Proline content in the assimilation apparatus of superabsorbent hydrogel-treated (SAH) and control seedlings in the greenhouse experiment for (A) oak, (B) beech, (C) spruce, and (D) pine. Horizontal lines indicate the mean, standard error (box), and minimum and maximum values (whiskers); KW–H represents the Kruskal–Wallis test results, significant differences are in bold.
3.2.2 Field experiment
No statistical differences in proline content were found in the trees in the field experiment. The physiological traits showed significantly higher variability in the measurements. Nevertheless, spruce (Figure 4C) had the highest proline concentration among the tree species, following the same trend observed in the greenhouse experiment, with the proline content of SAH-treated seedlings being higher than that of the control. The proline content of SAH-treated beech was also slightly higher than that of the control (Figure 4B), as was also the case for pine (Figure 4D). The proline content in oak was almost untraceable (Figure 4A), ranging from 0.02 to 0.04 ± 0.01 μmol g−1 of FW in both groups.
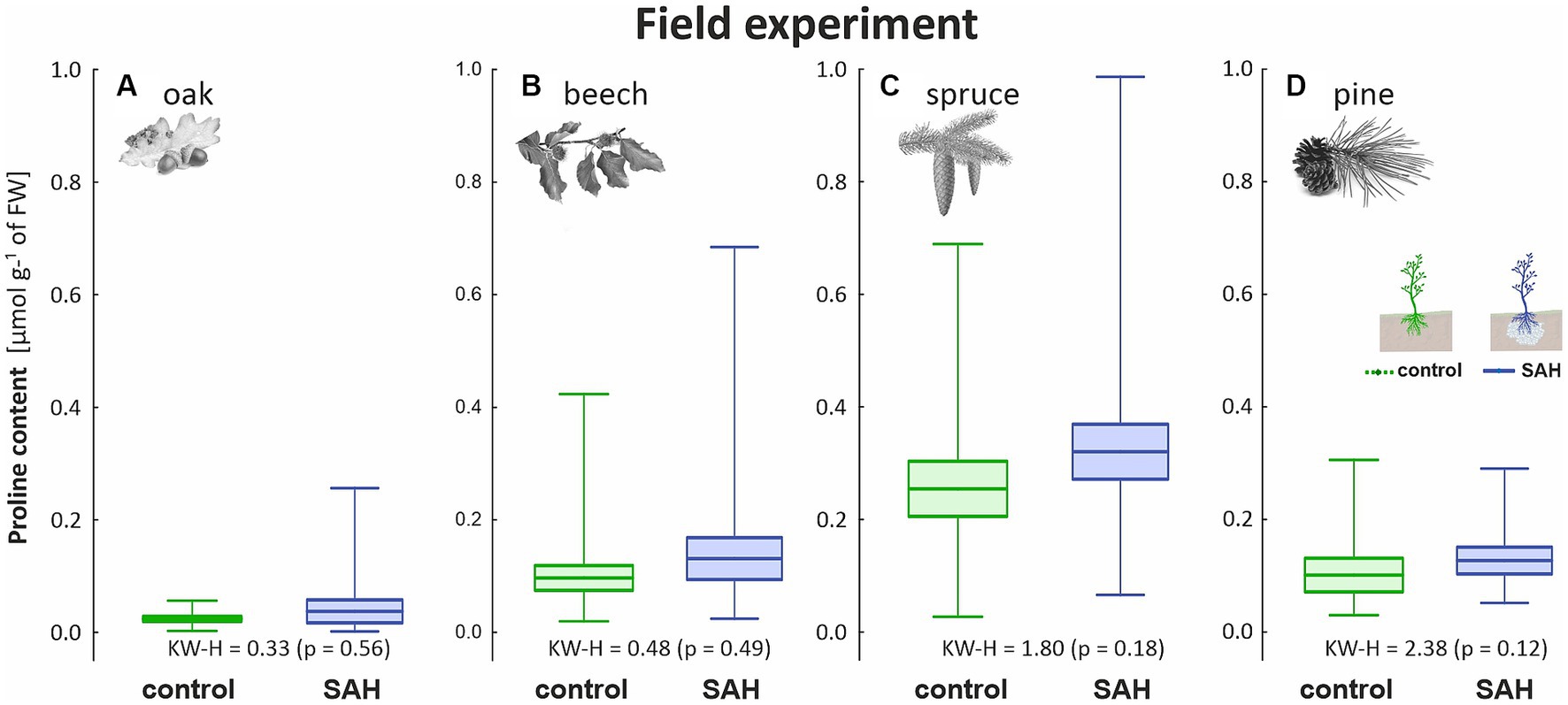
Figure 4. Proline content in the assimilation apparatus of superabsorbent hydrogel-treated (SAH) and control seedlings in the field experiment for (A) oak, (B) beech, (C) spruce, and (D) pine. Horizontal lines indicate the mean, box indicates the standard error and whiskers indicate the minimum and maximum values; KW–H represents Kruskal–Wallis test results—significant differences are in bold.
3.3 Morphological parameters
3.3.1 Greenhouse experiment
Overall, the seedlings exhibited excellent vitality during the experiment (Table 1), with only pine seedlings exhibiting reduced vitality and increased mortality. Seedling mortality was 8% higher in the control than in the SAH group. Moreover, only the SAH-treated pine had significantly lower height and thickness increments compared with the control group. For the other tree species, there was no significant difference in the measured morphological parameters between the control and SAH-treated seedlings (Table 1).
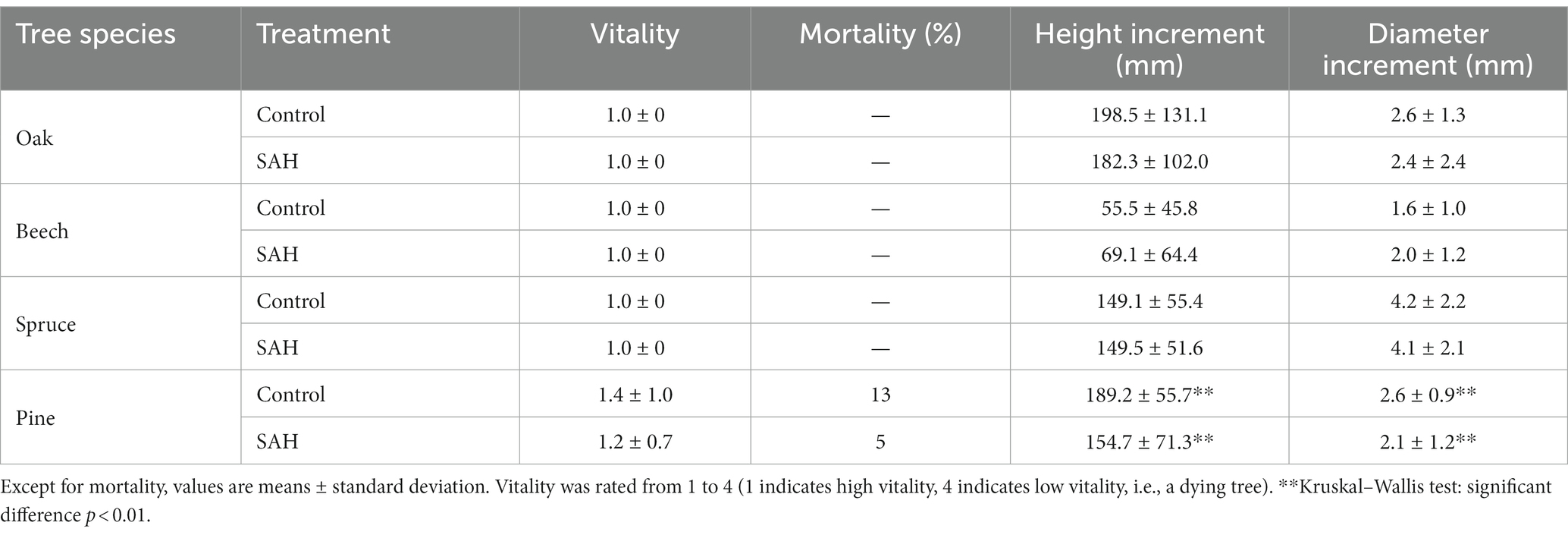
Table 1. Vitality, mortality, height increment, and diameter increment of four tree species during the 2020 growing season in the greenhouse.
3.3.2 Field experiment
The seedlings in the field experiment generally exhibited lower vitality, higher mortality, and lower height increments than those in the greenhouse experiment, unrelated to the applied treatment (Table 2). The application of SAH to seedling species resulted in a decrease in the mortality rates of all four tree species, with decreases of 4%, 6%, 1%, and 9% in oak, beech, spruce, and pine, respectively. The overall vitality of the seedlings was comparable among the SAH-treated and control groups. Only significant improvements in vitality were observed in beech seedlings. Additionally, a significantly greater height increment in the SAH-treated seedlings of beech was observed (Table 2). There were no other significant differences recorded in the morphological parameters of the studied species between the control and SAH-treated seedlings (Table 2).
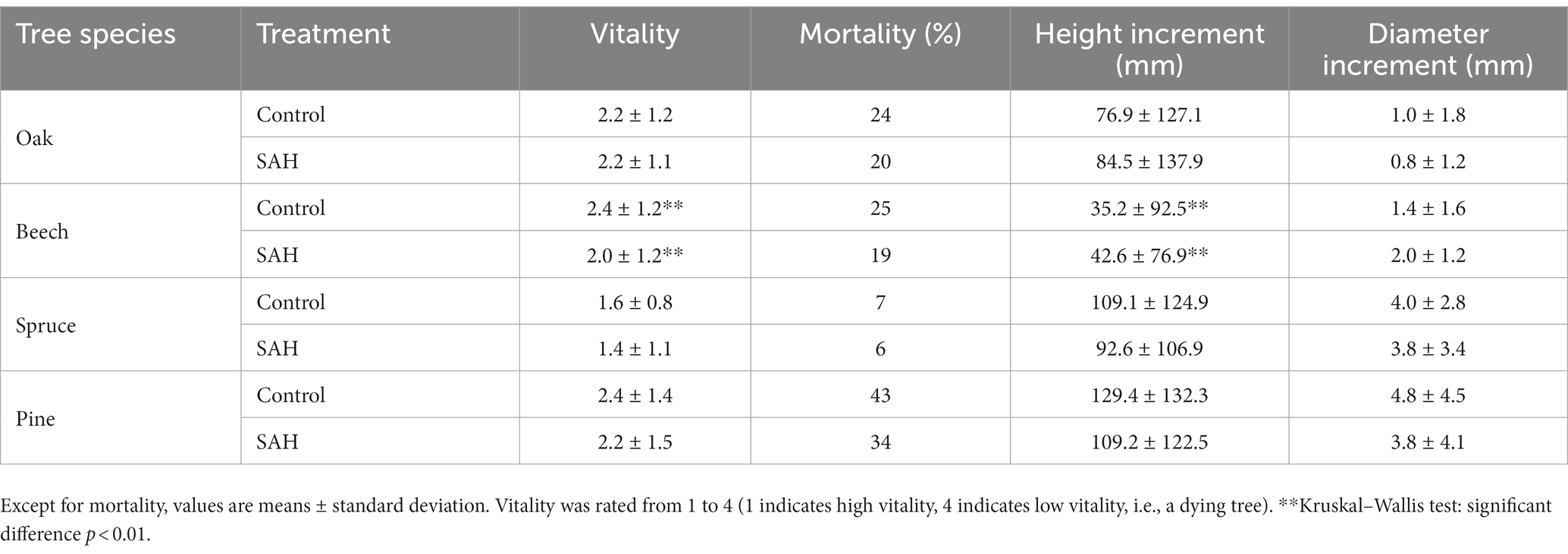
Table 2. Vitality, mortality, height increment, and diameter increment of four tree species during the 2020 growing season in the field.
4 Discussion
Positive effects of hydrogel and soil amendment on growth, water-use efficiency, and dry matter production of seedlings are well documented (Vierro and Little, 2006; Thomas, 2008; Kumar et al., 2020; Tomášková et al., 2020); however, uncertainties still persist due to the number of experiments that have also shown either no or even negative impacts (Oscroft, 2000; Del Campo et al., 2011). In addition, knowledge of the effect of SAH on trees in the absence of drought stress is still lacking, with research focusing on a small spectrum of tree species and climatic conditions (Orikiriza et al., 2009). Our findings suggest that, under a favorable climate, the studied European tree species did not show a significant response to SAH treatment, with only oak responding positively regarding its physiological parameters.
The long-term effects of synthetic hydrogels on ecosystems remains an important issue, mainly the process of the polymerization of acrylic acid, which could potentially lead to negative impacts on soil microflora or threaten plant health. A higher concentration of sodium in hydrogels prevents calcium ion uptake, and plants can suffer from mineral deficiency (Jeyabaskaran et al., 2021). Moreover, maintaining high osmotic pressure might also negatively influence the water-absorbing power of roots (Jeyabaskaran et al., 2021). Sodium ions released the hydrogels should prevent water stress by synthesizing osmotic active substances, but may also cause osmotic stress to plants (Cea et al., 2022). Even though no adverse effect of synthetic hydrogel on soil microbiota has been reported, mitigation of the soil microbiota was recorded (Karagöz and Yücel, 2020). On the other hand, microbiota mitigation has been revealed to have a positive effect on delaying denitrification and thus preventing nitrogen loss from the ground (Wang et al., 2020). All these findings lead to the development of natural-based hydrogels and their application to the environment (Wang et al., 2020).
In this study, no negative response of seedlings to SAH presence was recorded, but given the relatively short duration of the experiments, the long-term effects of SAH remain uncertain.
4.1 Basic physiological parameters
Positive effects on the growth performance and physiological traits of plants using synthesized hydrogels under drought or dry conditions have been reported (Coello et al., 2018). In this study, SAH treatment had no effect on spruce, beech, and pine under a favorable climate (Figures 1, 2). These results support the idea of complicated relationships among the ecological demands of species, soil and environmental conditions. In the study by Sarvaš (2003), spruce, as a shallow-rooted species, profited greatly from the addition of SAH; however, pine planted in sandy soil did not respond to SAH treatments well (Sarvaš et al., 2007). Generally, hydrogel application is more beneficial for shallow-rooted trees than deep-rooted trees because water drainage is prevented below the root zone (Yu et al., 2012). On the other hand, the physiological performance of pine and beech treated with SAH is better pronounced in loamy and clay soils than in sandy soils (Crous, 2017).
The conflicting results mainly arise from interactions between tree species and soil types (Orikiriza et al., 2013), with soil type influencing tree survival in SAH-amended soil (Agaba et al., 2010). According to Crous (2017), the overall physiological performance of SAH-treated woody plants was more pronounced in sandy soils than in loamy soils. The discrepancies between the morphological and physiological results might be explained by the different distributions of carbon sinks above and below the ground. The differences between the field and greenhouse experiments arise from a variety of external factors that might have affected the results in the field experiment.
Other sources of conflicting results include the varying ecological demands of different tree species. Under semi-arid conditions, drought-sensitive species exhibited a positive response to SAH treatment compared with tree species that were less vulnerable to drought (Tomášková et al., 2020). The SAH-treated oaks, both in the field and greenhouse experiments, displayed the most positive response in assimilation rate (15%) among the four tree species in both groups (Figures 1, 2). The higher photosynthetic performance in oak treated with SAH could be explained by hydraulic conductivity. As hydraulic conductivity is closely related to stomatal conductance and CO2 uptake, it might be the reason for the higher transpiration and photosynthetic rates of oak under stable water conditions. In oak, the hydraulic conductivity reaches 5.8 kg m−2 s−1 MPa−1, while that in beech reaches only 4.4 kg m−2 s−1 MPa−1 (Aranda et al., 2005). Conifers have generally lower hydraulic conductivity due to the anatomical structure of conducting elements (Song et al., 2022). Moreover, they exhibit isohydric behavior, which causes a linear decrease in stomatal conductance with a decrease in hydraulic conductivity (Martínez-Sancho et al., 2017); in contrast, oak maintains high stomatal conductance over a wide range of hydraulic conductivity values (Hubbard et al., 2001). According to Jamnická et al. (2013), the WUE is closely related to drought and decreases with prolonged drought. With enough water, the conductive system of oak transfers a larger bulk of water than the other investigated species, supported by the significantly lower values of water use efficiency recorded in the SAH-treated oak seedlings (Figures 1D, 2D).
These complexities underscore the intricate web of factors shaping hydrogel–plant interactions, promising ongoing exploration and discovery. While conflicting outcomes arise from intricate tree–soil interactions and species-specific demands, the dominant narrative emphasizes the advantageous effects of hydrogels on the growth and physiological traits of oak.
4.2 Proline content
Proline accumulation occurs in the early stages of water deficit and contributes to the photoprotection and stabilization of photosynthesis, ultimately enhancing the drought tolerance of plants (Sivakumar et al., 2000). As proline metabolism is closely linked to photosynthetic electron transport, it may also reduce the risk of reactive oxygen species overproduction in adverse environments (Ben Rejeb et al., 2014). The proline content under non-stress conditions ranges from 0.5 to 2.0 μmol g−1 of FW (Jamnická et al., 2019), depending on the provenance. The biochemical analysis of our data revealed differences in proline content among the tree species. Spruce had the highest proline content, but was still below 1.0 μmol g−1 of FW, and only oak showed a higher proline content in the greenhouse experiment when treated with SAH (Figure 3A). However, these values are significantly lower than the values reported under stress conditions (Robakowski et al., 2020; Arab et al., 2022) and thus do not indicate a stress response.
4.3 Morphological parameters
The seedling vitality of the studied tree species was comparable for the hydrogel treatment and the control (Tables 1, 2). The only exception was beech seedlings in the field experiment, which exhibited better vitality after SAH treatment. Seedling mortality was up to 9% higher in seedlings without hydrogel addition. This is consistent with previous studies that confirmed the prolonged survival time of seedlings in SAH-amended soil compared with the controls (Orikiriza et al., 2013) and generally, SAH application improved survival by 8%–18% (Crous, 2017). Furthermore, SAH applied under controlled conditions tended to have a higher frequency of positive survival responses compared with field experiments (Crous, 2017). This is consistent with our results where seedling mortality was only observed in pine; the other tree species in the greenhouse experiment thrived well regardless of treatment (Table 2).
Not only did SAH affect survival, but its positive effects on the height and diameter increments of tree seedlings have also been reported (Sarvaš, 2003; Vierro and Little, 2006; Sarvaš et al., 2007; Šijačić-Nikolić et al., 2011; Tomášková et al., 2020). Surprisingly, our results only showed significant differences in seedling growth for pine in the greenhouse experiment (both height and diameter increment) and for beech in the field experiment (height increment), with only beech having a positive response to SAH (Tables 1, 2).
Non-significant effects of SAH on the seedling growth of several tree species, e.g., spruce and beech (Repáč et al., 2013), cork oak (Chirino et al., 2011) or pines (Oscroft, 2000) have been already documented. Such conclusions underline the complex response of tree seedlings to SAH treatment, varying and depending not only on the tree species, soil conditions, and type or the amount of hydrogel added, but also on the method of application, quality of seedlings, and numerous external factors (Crous, 2017). Thus, the exact quantification of both positive and negative effects of SAH on plant growth remains challenging.
5 Conclusion
The assessment of the potential of SAH application in forest tree planting offers valuable insights into nursery management and planting strategies. However, the inconsistent research findings on the effects of SAH impede the drawing of definitive conclusions relevant to European forestry management. Our findings demonstrate that, in the absence of extended drought periods, applying SAH does not adversely affect economically valuable tree species. In contrast, it modestly enhanced their survival and physiological well-being, particularly in the case of oak. Considering its long-acting effect, SAH shows potential as an effective tool for pre-emptive measures and preparation for transplanting in case of prolonged drought periods in the coming years. Hydrogel treatment can be employed as a preventive measure in forest regeneration programs, particularly in Central Europe, where changing climatic conditions are expected to result in more frequent and severe drought events.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
IT, KR, and JM designed the study. KR and JM collected data from the field study. IT, JB, and FP performed the analytical methods in the laboratory. JT performed the statistical analyses and illustrated the graphics. IT, KR, and JT prepared the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Agency for Agricultural Research within the framework of projects under grant no. QK1910347 administered by the Ministry of Agriculture of the Czech Republic.
Acknowledgments
The authors would like to acknowledge our colleagues and students who helped us perform the experiments (Hana Vanická, Michal Svatoš, and Václav Štícha). The authors would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1251041/full#supplementary-material
References
Agaba, H., Orikiriza, L., Osoto Esegu, J. F., Obua, J., Kabasa, J. D., and Hüttermann, A. (2010). Effects of hydrogel amendment to different soils on plant available water and survival of trees under drought conditions. Clean 38, 328–335. doi: 10.1002/clen.200900245
Akhter, J., Mahmood, K., Malik, K. A., Mardan, A., Ahmad, M., and Iqbal, M. M. (2004). Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 50, 463–469. doi: 10.17221/4059-PSE
Alshallash, K. S., Sharaf, M., Hmdy, A. E., Khalifa, S. M., Abdel-Aziz, H. F., Sharaf, A., et al. (2022). Hydrogel improved growth and productive performance of mango trees under semi-arid condition. Gels 8:602. doi: 10.3390/gels8100602
Arab, L., Seegmueller, S., Kreuzwieser, J., Eiblmeier, M., Dannenmann, M., and Rennenberg, H. (2022). Significance of current weather conditions for foliar traits of old-growth sessile oak (Quercus petraea Liebl) trees. Trees 36, 777–791. doi: 10.1007/s00468-021-02249-x
Aranda, I., Gil, L., and Pardos, J. A. (2005). Seasonal changes in apparent hydraulic conductance and their implications for water use of European beech (Fagus sylvatica L.) and sessile oak [Quercus petraea (Matt.) Liebl] in South Europe. Plant Ecol. 179, 155–167. doi: 10.1007/s11258-004-7007-1
Ben Rejeb, K., Abdelly, C., and Savouré, A. (2014). How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80, 278–284. doi: 10.1016/j.plaphy.2014.04.007
Beniwal, R. S., Hooda, M. S., and Polle, A. (2011). Amelioration of planting stress by soil amendment with a hydrogel-mycorrhiza mixture for early establishment of beech (Fagus sylvatica L.) seedlings. Ann. For. Sci. 68, 803–810. doi: 10.1007/s13595-011-0077-z
Brázdil, R., Trnka, M., Řezníčková, L., Balek, J., Bartošová, L., Bičík, I., et al. (2015). Historie počasí a podnebí v českých zemích XI: Sucho v českých zemích: minulost, současnost a budoucnost. Brno: Centrum výzkumu globální změny Akademie věd České republiky
Cea, D., Bonomelli, C., Mártiz, J., and Gil, P. M. (2022). Response of potted citrus trees subjected to water deficit irrigation with the application of superabsorbent polyacrylamide polymers. Agronomy 12:1546. doi: 10.3390/agronomy12071546
Chirino, E., Vilagrosa, A., and Vallejo, V. R. (2011). Using hydrogel and clay to improve the water status of seedlings for dryland restoration. Plant Soil 344, 99–110. doi: 10.1007/s11104-011-0730-1
Choi, S., Lee, W., Kwak, D., Lee, S., Son, Y., Lim, J., et al. (2011). Predicting forest cover changes in future climate using hydrological and thermal indices in South Korea. Clim. Res. 49, 229–245. doi: 10.3354/cr01026
Coello, J., Ameztegui, A., Rovira, P., Fuentes, C., and Piqué, M. (2018). Innovative soil conditioners and mulches for forest restoration in semiarid conditions in Northeast Spain. Ecol. Eng. 118, 52–65. doi: 10.1016/j.ecoleng.2018.04.015
Crous, J. W. (2017). Use of hydrogels in the planting of industrial wood plantations. South. For. 79, 197–213. doi: 10.2989/20702620.2016.1221698
Del Campo, A. D., Hermoso, J., Flors, J., Lidon, A., and Navarro-Cerrillo, R. M. (2011). Nursery location and potassium enrichment in Aleppo pine stock 2. Performance under real and hydrogel-mediated drought conditions. Forestry 84, 235–245. doi: 10.1093/forestry/cpr009
Ekebafe, L. O., Ogbeifun, D. E., and Okieimen, F. E. (2011). Polymer applications in agriculture. Biokemistri 23, 81–89.
Hamdy, A., Khalifa, S., and AbdEl-Aziz, H. (2020). Hydrogel as a soil conditioner affecting the growth, yield, and fruit quality of ‘Murcott’ mandarin trees under arid and semi-arid lands. Al-Azhar J. Agric. Res. 45, 76–85. doi: 10.21608/ajar.2020.148791
Hlásny, T., Mátyás, C., Seidl, R., Kulla, L., Merganičová, K., Trombik, J., et al. (2014). Climate change increases the drought risk in Central European forests: what are the options for adaptation? For. J. 60, 5–18. doi: 10.2478/forj-2014-0001
Hubbard, R. M., Ryan, M. G., Stiller, V., and Sperry, J. S. (2001). Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 24, 113–121. doi: 10.1046/j.1365-3040.2001.00660.x
Hüttermann, A., Zommorodi, M., and Reise, K. (1999). Addition of hydrogels to soil for prolonging the survival of Pinus halepensis seedlings subjected to drought. Soil Tillage Res. 50, 295–304. doi: 10.1016/S0167-1987(99)00023-9
Jamnická, G., Ditmarová, Ľ., Kurjak, D., Kmeť, J., Pšidová, E., Macková, M., et al. (2013). The soil hydrogel improved photosynthetic performance of beech seedlings treated under drought. Plant Soil Environ. 59, 446–451. doi: 10.17221/170/2013-PSE
Jamnická, G., Fleischer, P. Jr., Konôpková, A., Pšidová, E., Kučerová, J., Kurjak, D., et al. (2019). Norway spruce (Picea abies L.) provenances use different physiological strategies to cope with water deficit. Forests 10:651. doi: 10.3390/f10080651
Jeyabaskaran, K. J., Shirgure, P. S., Vikramaditya Pandey, A. K., and Srivastava, S. U. (2021). Fertigation in horticulture: a guarantee to economized quality production. Indian J. Fert. 17, 364–383.
Jungsinyatam, P., Suwanakood, P., and Saengsuwan, S. (2022). Multicomponent biodegradable hydrogels based on natural biopolymers as environmentally coating membrane for slow-release fertilizers: effect of crosslinker type. Sci. Total Environ. 843:157050. doi: 10.1016/J.SCITOTENV.2022.157050
Karagöz, İ., and Yücel, G. (2020). Use of super absorbent polymers with Euonymus plants (Euonymus japonicus “Aureomarginatus”) in ornamental plant cultivation. Tarım Bilimleri Dergisi 26, 201–211. doi: 10.15832/ankutbd.471855
Kargar, M., Suresh, R., Legrand, M., Jutras, P., Clark, O. G., and Prasher, S. O. (2017). Reduction in water stress for tree saplings using hydrogels in soil. J. Geosci. Environ. Prot. 5, 27–39. doi: 10.4236/gep.2017.51002
Koupai, J. A., Eslamian, S. S., and Kazemi, J. A. (2008). Enhancing the available water content in unsaturated soil zone using hydrogel, to improve plant growth indices. Ecohydrol. Hydrobiol. 8, 67–75. doi: 10.2478/V10104-009-0005-0
Kumar, R., Yadav, S., Singh, V., Kumar, M., and Kumar, M. (2020). Hydrogel and its effect on soil moisture status and plant growth: a review. J. Pharmacogn. Phytochem. 9, 1746–1753.
Lee, M. R., Kim, C. S., Park, T., Choi, Y.-S., and Lee, K.-H. (2018). Optimization of the ninhydrin reaction and development of a multiwell plate-based high-throughput proline detection assay. Anal. Biochem. 556, 57–62. doi: 10.1016/j.ab.2018.06.022
Maitah, M., Malec, K., and Maitah, K. (2021). Influence of precipitation and temperature on maize production in the Czech Republic from 2002 to 2019. Sci. Rep. 11:10467. doi: 10.1038/s41598-021-89962-2
Marchi, E., Chung, W., Visser, R., Abbas, D., Nordfjell, T., Mederski, P. S., et al. (2018). Sustainable forest operations (SFO): a new paradigm in a changing world and climate. Sci. Total Environ. 634, 1385–1397. doi: 10.1016/J.SCITOTENV.2018.04.084
Martínez-Sancho, E., Vásconez Navas, L. K., Seidel, H., Dorado-Liñán, I., and Menzel, A. (2017). Responses of contrasting tree functional types to air warming and drought. Forests 8:450. doi: 10.3390/f8110450
Orikiriza, L. J. B., Agaba, H., Eilu, G., Kabasa, J. D., Worbes, M., and Hüttermann, A. (2013). Effects of hydrogels on tree seedling performance in temperate soils before and after water stress. J. Environ. Prot. 4, 713–721. doi: 10.4236/jep.2013.47082
Orikiriza, L. J. B., Agaba, H., Tweheyo, M., Eilu, G., Kabasa, J. D., and Hüttermann, A. (2009). Amending soils with hydrogels increases the biomass of nine tree species under non-water stress conditions. Clean 37, 615–620. doi: 10.1002/clen.200900128
Oscroft, D. G. (2000). The effect of a soil-amended hydrogel on the establishment of Pinus elliottii × caribaea rooted cuttings on the Zululand coastal sand. Pietermaritzburg: Institute for Commercial Forestry Research.
Podrázský, V., Remeš, J., Hart, V., and Moser, W. K. (2009). Production and humus form development in forest stands established on agricultural lands—Kostelec nad Černými lesy region. J. For. Sci. 55, 299–305. doi: 10.17221/11/2009-JFS
Repáč, I., Kmeť, J., Vencurik, J., and Balanda, M. (2013). Účinky aplikácie komerčných stimulačných prípravkov na prežívanie, rastové a fyziologické parametre výsadby smreka obyčajného a buka lesného. Zprávy lesnického výzkumu 58, 167–175.
Robakowski, P., Wyka, T. P., Kowalkowski, W., Barzdajn, W., Pers-Kamczyc, E., Jankowski, A., et al. (2020). Practical implications of different phenotypic and molecular responses of evergreen conifer and broadleaf deciduous forest tree species to regulated water deficit in a container nursery. Forests 11:1011. doi: 10.3390/f11091011
Rydlová, J., and Püschel, D. (2020). Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 146:103394. doi: 10.1016/j.apsoil.2019.103394
Sáenz-Romero, C., O’Neill, G., Aitken, S. N., and Lindig-Cisneros, R. (2020). Assisted migration field tests in Canada and Mexico: lessons, limitations, and challenges. Forests 12:9. doi: 10.3390/f12010009
Saha, A., Sekharan, S., and Manna, U. (2020). Superabsorbent hydrogel (SAH) as a soil amendment for drought management: a review. Soil Tillage Res. 204:104736. doi: 10.1016/J.STILL.2020.104736
Sarvaš, M. (2003). Effect of desiccation on the root system of Norway spruce (Picea abies [L.] karst.) seedlings and a possibility of using hydrogel STOCKOSORB® for its protection. J. For. Sci. 49, 531–536. doi: 10.17221/4796-JFS
Sarvaš, M., Pavlenda, P., and Takáčová, E. (2007). Effect of hydrogel application on survival and growth of pine seedlings in reclamations. J. For. Sci. 53, 203–209. doi: 10.17221/2178-JFS
Šijačić-Nikolić, M., Vilotić, D., and Milovanović, J. (2011). Effect of polymers on Scots pine (Pinus silvestris L.) and Austrian pine (Pinus nigra Arn.) seedling development in afforestation. Glob. J. Biodiver. Sci. Manag. 1, 11–18.
Sivakumar, P., Sharmila, P., and Pardha Saradhi, P. (2000). Proline alleviates salt-stress-induced enhancement in ribulose-1,5-bisphosphate oxygenase activity. Biochem. Biophys. Res. Commun. 279, 512–515. doi: 10.1006/bbrc.2000.4005
Song, Y., Sterck, F., Zhou, X., Liu, Q., Kruijt, B., and Poorter, L. (2022). Drought resilience of conifer species is driven by leaf lifespan but not by hydraulic traits. New Phytol. 235, 978–992. doi: 10.1111/nph.18177
Thomas, D. S. (2008). Hydrogel applied to the root plug of subtropical eucalypt seedlings halves transplant death following planting. For. Ecol. Manag. 255, 1305–1314. doi: 10.1016/j.foreco.2007.10.035
Tomášková, I., Svatoš, M., Macků, J., Vanická, H., Resnerová, K., Čepl, J., et al. (2020). Effect of different soil treatments with hydrogel on the performance of drought-sensitive and tolerant tree species in a semi-arid region. Forests 11:211. doi: 10.3390/f11020211
Vierro, P. V. M., and Little, K. M. (2006). A comparison of different planting methods, including hydrogels, and their effect on eucalypt survival and initial growth in South Africa. South. Afr. For. J. 208, 5–13. doi: 10.2989/10295920609505256
Wang, Z., Geng, Y., and Liang, T. (2020). Optimization of reduced chemical fertilizer use in tea gardens based on the assessment of related environmental and economic benefits. Sci. Total Environ. 713:136439. doi: 10.1016/j.scitotenv.2019.136439
Watanabe, K., Saensupo, S., Na-iam, Y., Klomsa-ard, P., and Sriroth, K. (2019). Effects of superabsorbent polymer on soil water content and sugarcane germination and early growth in sandy soil conditions. Sugar Tech 21, 444–450. doi: 10.1007/s12355-018-0672-5
Yu, J., Shi, J. G., Dang, P. F., Mamedov, A. I., Shainberg, I., and Levy, G. J. (2012). Soil and polymer properties affecting water retention by superabsorbent polymers under drying conditions. Soil Sci. Soc. Am. J. 76, 1758–1767. doi: 10.2136/sssaj2011.0387
Keywords: soil conditioners, carbon dioxide assimilation, fast kinetics of fluorescence, transpiration, vitality, morphology
Citation: Tomášková I, Resnerová K, Trombik J, Bláha J, Pastierovič F and Macků J (2023) Potential of hydrogel treatment in forest regeneration: impact on growth and vitality of Central European tree species. Front. For. Glob. Change. 6:1251041. doi: 10.3389/ffgc.2023.1251041
Edited by:
Martin D. Venturas, Universidad Politécnica de Madrid, SpainReviewed by:
Johanna Witzell, Linnaeus University, SwedenBaoguo Du, Mianyang Normal University, China
Copyright © 2023 Tomášková, Resnerová, Trombik, Bláha, Pastierovič and Macků. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karolina Resnerová, resnerovak@fld.czu.cz
 Ivana Tomášková
Ivana Tomášková Karolina Resnerová
Karolina Resnerová Jiří Trombik
Jiří Trombik